A kinetic study of [OsO4] reduction by aliphatic alcohols (MeOH and EtOH) was performed in a 2.0 M NaOH matrix at 298.1 K. The rate model that best fitted the UV-VIS data supports a one-step, two electron reduction of OsVIII (present as both the [OsVIIIO4(OH)]− and cis-[OsVIIIO4(OH)2]2− species in a ratio of 0.34 : 0.66) to form the trans-[OsVIO2(OH)4]2− species. The formed trans-[OsVIO2(OH)4]2− species subsequently reacts relatively rapidly with the cis-[OsVIIIO4(OH)2]2− complex anion to form a postulated [OsVIIO3(OH)3]2− species according to: cis-[OsVIIIO4(OH)2]2− + trans-[OsVIO2(OH)4]2−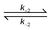 2[OsVIIO3(OH)3]2−. The calculated forward, k+2, and reverse, k−2, reaction rate constants of this comproportionation reaction are 620.9 ± 14.6 M−1s−1 and 65.7 ± 1.2 M−1s−1 respectively. Interestingly, it was found that the postulated [OsVIIO3(OH)3]2− complex anion does not oxidize MeOH or EtOH. Furthermore, the reduction of OsVIII with MeOH or EtOH is first order with respect to the aliphatic alcohol concentration. In order to corroborate the formation of the [OsVIIO3(OH)3]2− species predicted with the rate model simulations, several OsVIII/OsVI mole fraction and mole ratio titrations were conducted in a 2.0 M NaOH matrix at 298.1 K under equilibrium conditions. These titrations confirmed that the cis-[OsVIIIO4(OH)2]2− and trans-[OsVIO2(OH)4]2− species react in a 1 : 1 ratio with a calculated equilibrium constant, KCOM, of 9.3 ± 0.4. The ratio of rate constants k+2 and k−2 agrees quantitatively with KCOM, satisfying the principle of detailed balance. In addition, for the first time, the molar extinction coefficient spectrum of the postulated [OsVIIO3(OH)3]2− complex anion is reported.
2[OsVIIO3(OH)3]2−. The calculated forward, k+2, and reverse, k−2, reaction rate constants of this comproportionation reaction are 620.9 ± 14.6 M−1s−1 and 65.7 ± 1.2 M−1s−1 respectively. Interestingly, it was found that the postulated [OsVIIO3(OH)3]2− complex anion does not oxidize MeOH or EtOH. Furthermore, the reduction of OsVIII with MeOH or EtOH is first order with respect to the aliphatic alcohol concentration. In order to corroborate the formation of the [OsVIIO3(OH)3]2− species predicted with the rate model simulations, several OsVIII/OsVI mole fraction and mole ratio titrations were conducted in a 2.0 M NaOH matrix at 298.1 K under equilibrium conditions. These titrations confirmed that the cis-[OsVIIIO4(OH)2]2− and trans-[OsVIO2(OH)4]2− species react in a 1 : 1 ratio with a calculated equilibrium constant, KCOM, of 9.3 ± 0.4. The ratio of rate constants k+2 and k−2 agrees quantitatively with KCOM, satisfying the principle of detailed balance. In addition, for the first time, the molar extinction coefficient spectrum of the postulated [OsVIIO3(OH)3]2− complex anion is reported.
![Graphical abstract: A kinetic and thermodynamic study of the unexpected comproportionation reaction between cis-[OsVIIIO4(OH)2]2− and trans-[OsVIO2(OH)4]2− to form a postulated [OsVIIO3(OH)3]2− complex anion](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C1DT10290G&imageInfo.ImageIdentifier.Year=2011)
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 2[OsVIIO3(OH)3]2−. The calculated forward, k+2, and reverse, k−2, reaction rate constants of this comproportionation reaction are 620.9 ± 14.6 M−1s−1 and 65.7 ± 1.2 M−1s−1 respectively. Interestingly, it was found that the postulated [OsVIIO3(OH)3]2− complex anion does not oxidize
2[OsVIIO3(OH)3]2−. The calculated forward, k+2, and reverse, k−2, reaction rate constants of this comproportionation reaction are 620.9 ± 14.6 M−1s−1 and 65.7 ± 1.2 M−1s−1 respectively. Interestingly, it was found that the postulated [OsVIIO3(OH)3]2− complex anion does not oxidize ![Graphical abstract: A kinetic and thermodynamic study of the unexpected comproportionation reaction between cis-[OsVIIIO4(OH)2]2− and trans-[OsVIO2(OH)4]2− to form a postulated [OsVIIO3(OH)3]2− complex anion](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C1DT10290G&imageInfo.ImageIdentifier.Year=2011)

 Please wait while we load your content...
Please wait while we load your content...