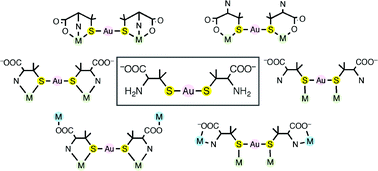
Developments in the rational creation of chiral multinuclear and metallosupramolecular compounds based on linear-type metal complexes with penicillaminate (pen), as well as their functionality as a multidentate chiral metalloligand, is the main subject of this paper. The reactions of a mononuclear AuI complex, [Au(D-pen)2]3−, in which two D-pen ligands bind to an AuI center through thiolato S atoms, with transition metal ions afford a variety of S-bridged heterobimetallic multinuclear complexes, the structures and properties of which are highly dependent on the nature of reacting metal ions. The created multinuclear complexes still act as a metalloligand when they possess free amine and/or carboxylate groups, leading to the formation of heterotrimetallic supramolecular structures by reacting with third metal ions. While the Au–S bonds in [Au(D-pen)2]3− are generally retained in the course of the reactions with metal ions, this is not the case for the Hg–S bonds in the corresponding HgII complex, [Hg(D-pen)2]2−. A remarkable chiral behavior of multinuclear complexes composed of [Au(L-cys)2]3− (cys = cysteinate), which is opposite to that composed of [Au(L-pen)2]3−, is also presented.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...