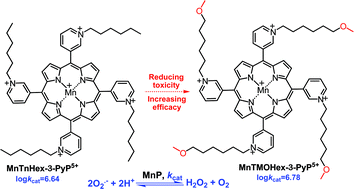
Cationic Mn(III) N-alkylpyridylporphyrins (MnPs) are potent SOD mimics and peroxynitrite scavengers and diminish oxidative stress in a variety of animal models of central nervous system (CNS) injuries, cancer, radiation, diabetes, etc. Recently, properties other than antioxidant potency, such as lipophilicity, size, shape, and bulkiness, which influence the bioavailability and the toxicity of MnPs, have been addressed as they affect their in vivo efficacy and therapeutic utility. Porphyrin bearing longer alkyl substituents at pyridyl ring, MnTnHex-2-PyP5+, is more lipophilic, thus more efficacious in vivo, particularly in CNS injuries, than the shorter alkyl-chained analog, MnTE-2-PyP5+. Its enhanced lipophilicity allows it to accumulate in mitochondria (relative to cytosol) and to cross the blood-brain barrier to a much higher extent than MnTE-2-PyP5+. Mn(III) N-alkylpyridylporphyrins of longer alkyl chains, however, bear micellar character, and when used at higher levels, become toxic. Recently we showed that meta isomers are ∼10-fold more lipophilic than ortho species, which enhances their cellular accumulation, and thus reportedly compensates for their somewhat inferior SOD-like activity. Herein, we modified the alkyl chains of the lipophilic meta compound, MnTnHex-3-PyP5+via introduction of a methoxy group, to diminish its toxicity (and/or enhance its efficacy), while maintaining high SOD-like activity and lipophilicity. We compared the lipophilic Mn(III) meso-tetrakis(N-(6′-methoxyhexyl)pyridinium-3-yl)porphyrin, MnTMOHex-3-PyP5+, to a hydrophilic Mn(III) meso-tetrakis(N-(2′-methoxyethyl)pyridinium-3-yl)porphyrin, MnTMOE-3-PyP5+. The compounds were characterized by uv-vis spectroscopy, mass spectrometry, elemental analysis, electrochemistry, and ability to dismute O2˙−. Also, the lipophilicity was characterized by thin-layer chromatographic retention factor, Rf. The SOD-like activities and metal-centered reduction potentials for the MnIIIP/MnIIP redox couple were similar-to-identical to those of N-alkylpyridyl analogs: log kcat = 6.78, and E1/2 = +68 mV vs. NHE (MnTMOHex-3-PyP5+), and log kcat = 6.72, and E1/2 = +64 mV vs. NHE (MnTMOE-3-PyP5+). The compounds were tested in a superoxide-specific in vivo model: aerobic growth of SOD-deficient E. coli, JI132. Both MnTMOHex-3-PyP5+ and MnTMOE-3-PyP5+ were more efficacious than their alkyl analogs. MnTMOE-3-PyP5+ is further significantly more efficacious than the most explored compound in vivo, MnTE-2-PyP5+. Such a beneficial effect of MnTMOE-3-PyP5+ on diminished toxicity, improved efficacy and transport across the cell wall may originate from the favorable interplay of the size, length of pyridyl substituents, rotational flexibility (the ortho isomer, MnTE-2-PyP5+, is more rigid, while MnTMOE-3-PyP5+ is a more flexible meta isomer), bulkiness and presence of oxygen.

 Please wait while we load your content...
Please wait while we load your content...