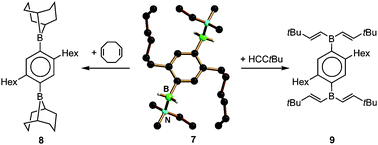
Single crystals of the meta- and para-phenylene-bridged ditopic trihydridoborates (Li(THF)2)2[m-C6H4(BH3)2] and (Li(THF)2)2[p-C6H4(BH3)2] have been prepared and investigated by X-ray crystallography. The compounds turned out to be coordination polymers in which each trihydridoborate substituent is connected with one trihydridoborate substituent of a neighbouring monomer via two bridging Li(THF)2+ ions. (Li(THF)2)2[m-C6H4(BH3)2] and (Li(THF)2)2[p-C6H4(BH3)2] suffer from poor solubility in all common non-protic solvents. Thus, a more soluble derivative of (Li(THF)2)2[p-C6H4(BH3)2], equipped with n-hexyl groups at the positions 2 and 5 of the phenylene ring, has been used for all further investigations (i.e., compound Li2[6]). Treatment of Li2[6] with Me3SiCl in the presence of excess N(Me)2Et leads to the abstraction of one hydride ion per boron atom under formation of the ditopic amine-borane adduct p-C6H2(n-hexyl)2(BH2–N(Me)2Et)2 (7). The compound turned out to be an efficient hydroboration reagent both for internal olefins (i.e., 1,5-cyclooctadiene) and terminal alkynes (i.e., tert-butyl acetylene) to give p-C6H2(n-hexyl)2(9-BBN)2 (8; 9-BBN = 9-borabicyclo[3.3.1]nonyl) and p-C6H2(n-hexyl)2(B(C(H)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)tBu)2)2 (9), respectively.
C(H)tBu)2)2 (9), respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)tBu)2)2 (9), respectively.
C(H)tBu)2)2 (9), respectively.

 Please wait while we load your content...
Please wait while we load your content...