Reaction of n-propylamino-N,N-bis(2-methylene-4-tert-butyl-6-methylphenol), H2L1, n-propylamino-N,N-bis(2-methylene-4,6-di-tert-butylphenol), H2L2, and benzylamino-N,N-bis(2-methylene-4-tert-butyl-6-methylphenol), H2L3, with anhydrous ferric chloride in the presence of base yields the products, [FeL1(μ-Cl)]2 (1), [FeL2(μ-Cl)]2 (2) and [FeL3(μ-Cl)]2 (3). In the solid state, these complexes exist as chloride-bridged dimers giving distorted trigonal bipyramidal iron(III) ions. Reaction of H2L1 with FeBr3, however, results in the formation of a tetrahedral iron(III) complex possessing two bromide ligands. The amine-bis(phenolate) ligand is bidentate in this complex and bonds to the iron(III) ion via the phenolate O-donors. The central amine donor is protonated, resulting in a quaternized ammonium fragment and the iron(III) centre possesses a negative formal charge. As a result, this complex is zwitterionic and formulated as FeBr2L1H (4). Complex 1 is an air-stable, non-hygroscopic, single-component catalyst for C–C cross-coupling of aryl Grignard reagents with primary and secondary alkyl halides, including chlorides. Good to excellent yields of cross-coupled products are obtained in diethyl ether at room temperature. In some cases where low yields are obtained under these conditions, the use of microwave-assisted heating of the reaction mixture can improve yields.
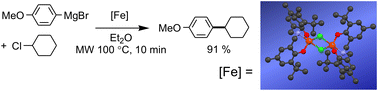
 Please wait while we load your content...
Please wait while we load your content...