Electron-deficient 1- and 2-azabuta-1,3-dienes: a comprehensive survey of their synthesis and reactivity
Abstract
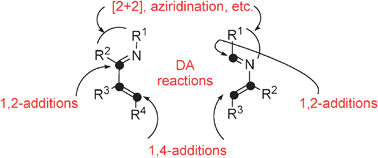
Electron-deficient 1- and 2-azabuta-1,3-dienes are reagents intrinsically able to provide a wide range of cyclic and acyclic N-containing building blocks. Depending on their substitution, they behave as dienes for Diels–Alder reactions, as partners for [4+1], [3+2], [2+2]-cycloadditions, for aziridinations and as electrophiles for 1,2 and 1,4-additions. Nowadays, they are a very versatile family of compounds, despite their usual instability and complex reactivity. Four decades of research in this challenging area are reviewed in this critical review: their synthetic aspects and their reactivity towards a wide range of dienophiles, dipoles and nucleophiles are described as well. The introduction focuses on their electronic properties in order to get a clear picture of their reactivity (190 references).

 Please wait while we load your content...
Please wait while we load your content...