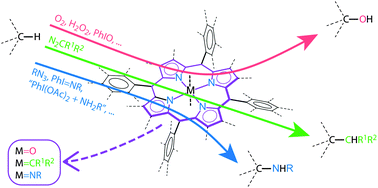
The recent surge of interest in metal-catalysed C–H bond functionalisation reactions reflects the importance of such reactions in biomimetic studies and organic synthesis. This critical review focuses on metalloporphyrin-catalysed saturated C–H bond functionalisation reported since the year 2000, including C–O, C–N and C–C bond formation via hydroxylation, amination and carbenoid insertion, respectively, together with a brief description of previous achievements in this area. Among the metalloporphyrin-catalysed reactions highlighted herein are the hydroxylation of steroids, cycloalkanes and benzylic hydrocarbons; intermolecular amination of steroids, cycloalkanes and benzylic or allylic hydrocarbons; intramolecular amination of sulfamate esters and organic azides; intermolecular carbenoid insertion into benzylic, allylic or alkane C–H bonds; and intramolecular carbenoid C–H insertion of tosylhydrazones. These metalloporphyrin-catalysed saturated C–H bond functionalisation reactions feature high regio-, diastereo- or enantioselectivity and/or high product turnover numbers. Mechanistic studies suggest the involvement of metal-oxo, -imido (or nitrene), and -carbene porphyrin complexes in the reactions. The reactivity of such metal–ligand multiple bonded species towards saturated C–H bonds, including mechanistic studies through both experimental and theoretical means, is also discussed (244 references).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...