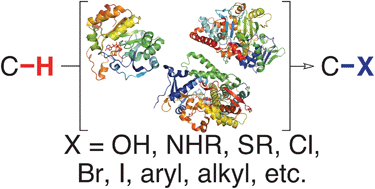
The development of new catalytic methods to functionalize carbon–hydrogen (C–H) bonds continues to progress at a rapid pace due to the significant economic and environmental benefits of these transformations over traditional synthetic methods. In nature, enzymes catalyze regio- and stereoselective C–H bond functionalization using transformations ranging from hydroxylation to hydroalkylation under ambient reaction conditions. The efficiency of these enzymes relative to analogous chemical processes has led to their increased use as biocatalysts in preparative and industrial applications. Furthermore, unlike small molecule catalysts, enzymes can be systematically optimized via directed evolution for a particular application and can be expressed in vivo to augment the biosynthetic capability of living organisms. While a variety of technical challenges must still be overcome for practical application of many enzymes for C–H bond functionalization, continued research on natural enzymes and on novel artificial metalloenzymes will lead to improved synthetic processes for efficient synthesis of complex molecules. In this critical review, we discuss the most prevalent mechanistic strategies used by enzymes to functionalize non-acidic C–H bonds, the application and evolution of these enzymes for chemical synthesis, and a number of potential biosynthetic capabilities uniquely enabled by these powerful catalysts (110 references).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...