Can an eight π-electron bare ring be planar?†
Abstract
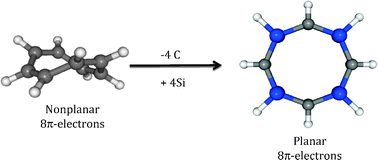
Here we explore in silico an alternative to make planar eight π-electron bare ring systems with substitutions of some cyclooctatetraene ring carbon atoms by heavier
- This article is part of the themed collection: Aromaticity, electron delocalisation, and related molecular properties

 Please wait while we load your content...
Please wait while we load your content...