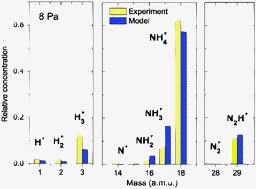
The chemistry in low pressure (0.8–8 Pa) plasmas of H2 + 10% N2 mixtures has been experimentally investigated in a hollow cathode dc reactor using electrical probes for the estimation of electron temperatures and densities, and mass spectrometry to determine the concentration of ions and stable neutral species. The analysis of the measurements by means of a kinetic model has allowed the identification of the main physicochemical mechanisms responsible for the observed distributions of neutrals and ions and for their evolution with discharge pressure. The chemistry of neutral species is dominated by the formation of appreciable amounts of NH3 at the metallic walls of the reactor through the successive hydrogenation of atomic nitrogen and nitrogen containing radicals. Both Eley–Rideal and Langmuir–Hinshelwood mechanisms are needed in the chain of hydrogenation steps in order to account satisfactorily for the observed ammonia concentrations, which, in the steady state, are found to reach values ∼30–70% of those of N2. The ionic composition of the plasma, which is entirely due to gas-phase processes, is the result of a competition between direct electron impact dissociation, more relevant for high electron temperatures (lower pressures), and ion–molecule chemistry that prevails for the lower electron temperatures (higher pressures). At the lowest pressure, products from the protonation of the precursor molecules (H3+, N2H+ and NH4+) and others from direct ionization (H2+ and NH3+) are found in comparable amounts. At the higher pressures, the ionic distribution is largely dominated by ammonium. It is found that collisions of H3+, NH3+ and N2H+ with the minor neutral component NH3 are to a great extent responsible for the final prevalence of NH4+.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...