Metastable ClO2+ and ClO3+ ions in the gas phase: a combined theoretical and mass spectrometric investigation
Abstract
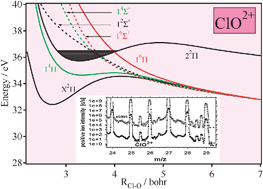
We have performed a detailed theoretical study applying large ab initio computations of the low lying electronic states of ClO2+ and ClO3+ and an experimental search for these ClO2+ and ClO3+ gas-phase species. For both species, we have found electronic states with potential barriers with respect to dissociation, where these multiply positively charged diatomic ions can exist in the gas phase as long-lived metastable molecules. Our potential energy curves are used to predict the double-ionization spectrum of ClO and to derive a set of spectroscopic parameters for the metastable bound states of these species. At the MRCI + Q/aug-cc-pV5Z level, the adiabatic double and triple ionization energies of ClO are computed to be 32.4 eV and 65.0 eV, respectively. Experimentally, we confirm the existence of ClO2+ using
- This article is part of the themed collection: Multiply charged ions (MCIs) in the gas-phase

 Please wait while we load your content...
Please wait while we load your content...