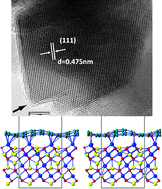
We investigate the effects of carbon coating, with and without nitrogen-dopants, on the electrochemical performance of a promising anode material Li4Ti5O12 (LTO) in lithium ion battery applications. The comparative experimental results show that LTO samples coated with nitrogen-doped carbon derived from pyridine and an ionic liquid exhibit significant improvements in rate capability and cycling performance compared with a LTO sample coated by carbon derived from toluene and the pristine LTO sample. For the first time, we construct an atomistic model for the interface between the lithium transition metal oxide and carbon coating layers. Our first-principles calculations based on density functional theory reveal that at this interface there is strong binding between the graphene coating layer and the Ti-terminated LTO surface, which significantly reduces the chemical activity of LTO surfaces and stabilizes the electrode/electrolyte interface, providing a clue to solve the swelling problem for LTO-based batteries. More importantly, electron transfer from the LTO surface to graphene greatly improves the electric conductivity of the interface. Nitrogen-dopants in graphene coatings further increase the interfacial stability and electric conductivity, which is beneficial to the electrochemical performance in energy storage applications.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...