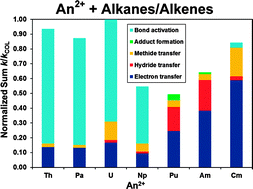
Small alkanes (methane, ethane, propane, n-butane) and alkenes (ethene, propene, 1-butene) were used to probe the gas-phase reactivity of doubly charged actinide cations, An2+ (An = Th, Pa, U, Np, Pu, Am, Cm), by means of Fourier transform ion cyclotron resonance mass spectrometry. Different combinations of doubly and singly charged ions were observed as reaction products, comprising species formed via metal-ion induced eliminations of small molecules, simple adducts and ions resulting from electron, hydride or methide transfer channels. Th2+, Pa2+, U2+ and Np2+ preferentially yielded doubly charged products of hydrocarbon activation, while Pu2+, Am2+ and Cm2+ reacted mainly through transfer channels. Cm2+ was also capable of forming doubly charged products with some of the hydrocarbons whereas Pu2+ and Am2+ were not, these latter two ions conversely being the only for which adduct formation was observed. The product distributions and the reaction efficiencies are discussed in relation to the electronic configurations of the metal ions, the energetics of the reactions and similar studies previously performed with doubly charged lanthanide and transition metal cations. The conditions for hydrocarbon activation to occur as related to the accessibility of electronic configurations with one or two 5f and/or 6d unpaired electrons are examined and the possible chemical activity of the 5f electrons in these early actinide ions, particularly Pa2+, is considered.

 Please wait while we load your content...
Please wait while we load your content...