In this work, we substantiate the change in the emitting state of indole caused by the dipolarity increase in the solvent 1-chlorobutane, observed on lowering the temperature from 293 to 133 K, accompanied by no significant changes in the corresponding excitation and absorption spectra. No similar changes in indole emission were observed over the temperature range 293–133 K for solutions of indole in 2-methylbutane in the presence and absence of 0.5 M 1-chlorobutane. The solvatochromism of indole in 1-chlorobutane at temperatures from 293 to 133 K allowed us to estimate the dipole moment and polarizability of the emission state of the chromophore and to detect two states (S1 and 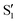 ): one, the S1, involving no significant change and the other, the
): one, the S1, involving no significant change and the other, the 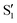 , exhibiting a substantial change in the dipole moment of the chromophore upon electronic excitation (viz. μS1 = 2.5 and
, exhibiting a substantial change in the dipole moment of the chromophore upon electronic excitation (viz. μS1 = 2.5 and 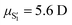 vs. μS0 = 2.13 D). The former state, S1, is the major contributor to the structured emission of indole at temperatures from 293 to 193 K, as is the latter,
vs. μS0 = 2.13 D). The former state, S1, is the major contributor to the structured emission of indole at temperatures from 293 to 193 K, as is the latter, 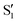 , to its structureless, red-shifted emission over the range 193–133 K. Although the emission changes of indole, dissolved in 1-chlorobutane at temperatures from 293 to 133 K, are seemingly consistent with the widely accepted photophysical model for inversion of its 1Lb and 1La states as the polarity of the medium is increased, below 133 K the emission becomes structured and blue-shifted, two typical features of indole above 193 K. Also, below 123 K is not feasible to photo-select the 1La state in spite of this state being the first excited electronic state of indole under large dipolarity conditions. Therefore, the established photophysical model cannot hold under these conditions and a new one accounting for these experimental facts is proposed instead.
, to its structureless, red-shifted emission over the range 193–133 K. Although the emission changes of indole, dissolved in 1-chlorobutane at temperatures from 293 to 133 K, are seemingly consistent with the widely accepted photophysical model for inversion of its 1Lb and 1La states as the polarity of the medium is increased, below 133 K the emission becomes structured and blue-shifted, two typical features of indole above 193 K. Also, below 123 K is not feasible to photo-select the 1La state in spite of this state being the first excited electronic state of indole under large dipolarity conditions. Therefore, the established photophysical model cannot hold under these conditions and a new one accounting for these experimental facts is proposed instead.
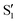 ): one, the S1, involving no significant change and the other, the
): one, the S1, involving no significant change and the other, the 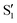 , exhibiting a substantial change in the dipole moment of the
, exhibiting a substantial change in the dipole moment of the 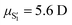 vs. μS0 = 2.13 D). The former state, S1, is the major contributor to the structured emission of
vs. μS0 = 2.13 D). The former state, S1, is the major contributor to the structured emission of 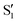 , to its structureless, red-shifted emission over the range 193–133 K. Although the emission changes of
, to its structureless, red-shifted emission over the range 193–133 K. Although the emission changes of 

 Please wait while we load your content...
Please wait while we load your content...