Electronic structure and oxygen vacancies in PdO and ZnO: validation of DFT models
Abstract
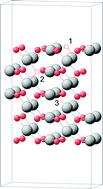
PdO is one of the most important catalytic materials currently used in the industry. In redox catalytic reactions involving PdO, the bulk phase is an additional source of oxygen. This leads to strong transformations not only at the surface of PdO but also in the near sub-surface and bulk regions. The redox process is, therefore, governed not only by the extent of PdO d-band filling, but also depends on the material properties of the PdO crystal—the ease with which its structure can be deformed. Methane

 Please wait while we load your content...
Please wait while we load your content...