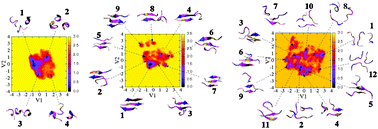
The aim of this work is to investigate the effects of molecular mechanics force fields on amyloid peptide assembly. To this end, we performed extensive replica exchange molecular dynamics (REMD) simulations on the monomer, dimer and trimer of the seven-residue fragment of the Alzheimer's amyloid-β peptide, Aβ16–22, using the AMBER99, GROMOS96 and OPLS force fields. We compared the force fields by analysing the resulting global and local structures as well as the free energy landscapes at 300 K. We show that AMBER99 strongly favors helical structures for the monomer and does not predict any β-sheet structure for the dimer and trimer. In contrast, the dimer and trimer modeled by GROMOS96 form antiparallel β-sheet structures, while OPLS predicts diverse structures. Overall, the free energy landscapes obtained by three force fields are very different, and we also note a weak structural dependence of our results on temperature. The implications of this computational study on amyloid oligomerization, fibril growth and inhibition are also discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...