High temperatures enhance cooperative motions between CBM and catalytic domains of a thermostable cellulase: mechanism insights from essential dynamics†
Abstract
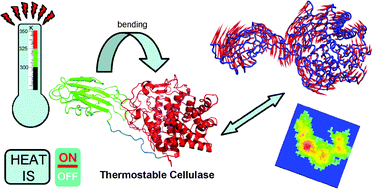
Cellulases from thermophiles are capable of cleaving sugar chains from cellulose efficiently at high temperatures. The thermo-resistant Cel9A-68 cellulase possesses two important domains: CBM and a catalytic domain connected by a Pro/Ser/Thr rich linker. These domains act cooperatively to allow efficient catalysis. Despite exhaustive efforts to characterize cellulase binding and mechanism of action, a detailed description of the cellulose intrinsic flexibility is still lacking. From computational simulations we studied the temperature influence on the enzyme plasticity, prior to substrate binding. Interestingly, we observed an enhancement of collective motions at high temperatures. These motions are the most representative and describe an intrinsic hinge bending transition. A detailed analysis of these motions revealed an interdomain approximation where D459 and G460, located at the linker region, are the hinge residues. Therefore, we propose a new putative site for mutagenesis targeting the modulation of such conformational transition that may be crucial for activity.

 Please wait while we load your content...
Please wait while we load your content...