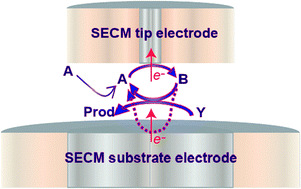
This paper describes the use of scanning electrochemical microscopy (SECM) in the tip generation/substrate collection (TG/SC), or feedback, mode and substrate generation/tip collection (SG/TC) mode to measure homogeneous kinetics in the catalytic EC′ process. Theoretical analyses of both configurations have been developed numerically to allow the optimal conditions for sensitive kinetic measurements to be determined. This is shown to involve collection efficiency measurements as a function of tip-substrate electrode distance in the case of TG/SC measurements and tip (collector current) images in a plane normal to the substrate electrode for the SG/TC mode. An important consideration for the SECM configuration (particularly for TG/SC and feedback measurements) is that the electroinactive co-reactant may be depleted more significantly than with other electrode geometries, because of cycling of the redox couple in the tip/substrate electrode gap, while the co-reactant can only enter this gap by hindered diffusion. The approaches described are examined through studies of the oxidation of amidopyrine by electrogenerated Fe(CN)3−6 in 0.5 mol dm−3 aqueous KOH solution. A second-order rate constant of 390 ± 80 dm3 mol−1 s−1 is obtained from TG/SC measurements, consistent with SG/TC quantitative imaging measurements. The consistency of the kinetic measurements confirms the validity of the approaches described. The kinetic constant is lower than expected based on previous ultramicroelectrode (UME) studies, and this is attributed to the fact that background currents for the direct heterogeneous oxidation of amidopyrine are more significant with conventional UME measurements, which will tend to enhance the current measured and may therefore lead to an overestimation of kinetic constants. The TG/SC approach, on the other hand, provides a means of making dual-electrode collection efficiency measurements with diffusional feedback of the redox couple, leading to superior voltammetric responses and enabling more accurate kinetic determination.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...