Nanoaggregate shapes at the air/water interface
Abstract
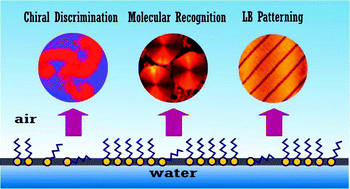
Chiral interfaces and molecular recognition phenomena are of special interest not only for the understanding of biological recognition processes but also for the potential application in material science. Langmuir monolayers at the air–
- This article is part of the themed collection: Materials innovation through interfacial physics and chemistry

 Please wait while we load your content...
Please wait while we load your content...