Electrocatalytic oxygen evolution from water on a Mn(iii–v) dimer model catalyst—A DFT perspective†
Abstract
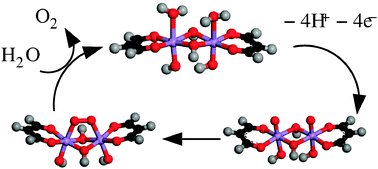
A complete water ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/Mn(IV)–O˙ moieties, which subsequently combine to form a μ-peroxy O–O bond. O2 evolution results from subsequent consecutive replacement of the remaining Mn–O bonds by
O/Mn(IV)–O˙ moieties, which subsequently combine to form a μ-peroxy O–O bond. O2 evolution results from subsequent consecutive replacement of the remaining Mn–O bonds by

 Please wait while we load your content...
Please wait while we load your content...