*
Corresponding authors
a
ICYS, International Center for Materials Nanoarchitectonics (MANA), 1-1 Namiki, Tsukuba, Japan
E-mail:
LEE.Michael@nims.go.jp
Fax: + 81 29 860 4706
Tel: + 81 29 851 3354
b
Max Planck Institute for Polymer Research, Polymer Theory, Ackermannweg 10, D 55128 Mainz, Germany
E-mail:
scipioni@mpip-mainz.mpg.de
Fax: + 49 6131 340
Tel: + 49 6131 379
c
Institut de Physique et Chimie des Matériaux de Strasbourg, UMR 7504 CNRS-UDS, 23 rue du Loess, BP 43, F-67034 Strasbourg, France
E-mail:
boero@ipcms.u-strasbg.fr
Fax: + 33 3 88 10 72 47
Tel: + 33 3 88 10 71 42
d
CREST, Japan Science and Technology Agency, Sanban-cho, Tokyo 102-0075, Japan
e
Japan Research Center for Integrated Science, Japan Advanced Institute of Science and Technology (JAIST), 1-1 Asahidai, Nomi, Ishikawa 923-1292, Japan
f
Dipartimento di Fisica, Universita' di Padova, Via Marzolo 8, Padova, Italy
E-mail:
psil@pd.infn.it
Fax: + 39 049 827 7102
Tel: + 39 049 827 7171
g
International Center for Materials Nanoarchitectonics (MANA), 1-1 Namiki, Tsukuba, Japan
E-mail:
ARIGA.Katsuhiko@nims.go.jp
Fax: + 81 29 860 4706
Tel: + 81 29 860 4832
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. On the silicon surface, however, the reaction proceeds efficiently at 200 °C, initiated by visible light, and more slowly at room temperature in the dark. Such low
C double bond. On the silicon surface, however, the reaction proceeds efficiently at 200 °C, initiated by visible light, and more slowly at room temperature in the dark. Such low 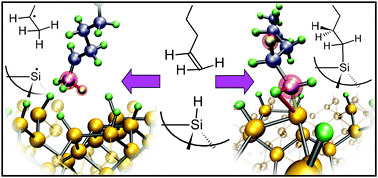

 Please wait while we load your content...
Please wait while we load your content...