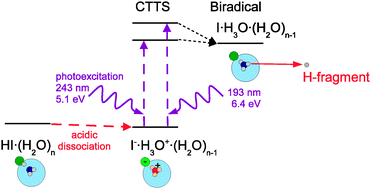
Photochemistry of HI molecules on large Arn and (H2O)n, ![[n with combining macron]](https://www.rsc.org/images/entities/i_char_006e_0304.gif) ∼ 100–500, clusters was investigated after excitation with 243 nm and 193 nm laser radiation. The measured H-fragment kinetic energy distributions pointed to a completely different photodissociation mechanism of HI on water than on argon clusters. Distinct features corresponding to the fragment caging (slow fragments) and direct exit (fast fragments) were observed in the spectra from HI photodissociation on Arnclusters. On the other hand, the fast fragments were entirely missing in the spectrum from HI·(H2O)n and the slow-fragment part of the spectrum had a different shape from HI·Arn. The HI·(H2O)nspectrum was interpreted in terms of the acidic dissociation of HI on (H2O)n in the ground state, and hydronium radical H3O formation following the UV excitation of the ionically dissociated species into states of a charge-transfer-to-solvent character. The H3O generation was proved by experiments with deuterated species DI and D2O. The experiment was complemented by ab initio calculations of structures and absorption spectra for small HI·(H2O)nclusters, n = 0–5, supporting the proposed model.
∼ 100–500, clusters was investigated after excitation with 243 nm and 193 nm laser radiation. The measured H-fragment kinetic energy distributions pointed to a completely different photodissociation mechanism of HI on water than on argon clusters. Distinct features corresponding to the fragment caging (slow fragments) and direct exit (fast fragments) were observed in the spectra from HI photodissociation on Arnclusters. On the other hand, the fast fragments were entirely missing in the spectrum from HI·(H2O)n and the slow-fragment part of the spectrum had a different shape from HI·Arn. The HI·(H2O)nspectrum was interpreted in terms of the acidic dissociation of HI on (H2O)n in the ground state, and hydronium radical H3O formation following the UV excitation of the ionically dissociated species into states of a charge-transfer-to-solvent character. The H3O generation was proved by experiments with deuterated species DI and D2O. The experiment was complemented by ab initio calculations of structures and absorption spectra for small HI·(H2O)nclusters, n = 0–5, supporting the proposed model.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[n with combining macron]](https://www.rsc.org/images/entities/i_char_006e_0304.gif) ∼ 100–500, clusters was investigated after excitation with 243 nm and 193 nm laser radiation. The measured H-fragment kinetic energy distributions pointed to a completely different photodissociation mechanism of HI on
∼ 100–500, clusters was investigated after excitation with 243 nm and 193 nm laser radiation. The measured H-fragment kinetic energy distributions pointed to a completely different photodissociation mechanism of HI on 

 Please wait while we load your content...
Please wait while we load your content...