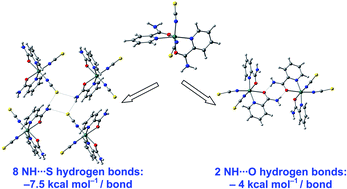
The new heteroleptic complexes, [M(NCS)2(pia)2] M = Co(II) (1), Ni(II) (2), and [Cu(SCN)2(pia)2] (3), pia = pyridine-2-carboxamide, were synthesized and characterized. Their single crystal X-ray diffraction structures showed octahedral units, with the two thiocyanate ligands occupying cis-positions and binding through the nitrogen atom in Co(II) and Ni(II). In the Cu(II) complex, they were trans and S-bound. The crystal structures display a 2-d structure with NH⋯S hydrogen bonds for the Co(II) and Ni(II), forming tetrameric units, and are isomorphous with the Zn(II) complex, [Zn(NCS)2(pia)2], and a 2-d structure with NH⋯N for the Cu(II). DFT calculations were performed on the new complexes and the analogous polymorphs of [Zn(NCS)2(pia)2], including a second one containing dimeric motifs. The calculated vibrational modes of the thiocyanate ligands corroborate the experimental ones and reflect the coordination mode of the ligand. A comparison between the two Zn(II) polymorphs showed that the NH⋯S bond in the tetramer is stronger (−7.50 kcal mol−1 per metal) than the NH⋯O bond in the dimer (−4.01 kcal mol−1 per metal), indicating a preference for the formation of tetrameric units. The NH⋯N hydrogen bonds calculated in the Cu(II) crystal are stronger (−9.15 kcal mol−1 per metal) than the NH⋯S ones in Ni(II) and Zn(II).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...