Catalytic enantioselective Grignard Nozaki–Hiyama methallylation from the alcohol oxidation level: chloride compensates for π-complex instability‡†
Abstract
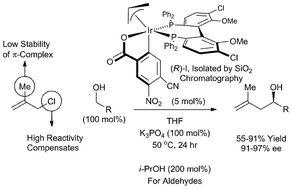
- This article is part of the themed collection: New advances in catalytic C–C bond formation via late transition metals

 Please wait while we load your content...
Please wait while we load your content...