A synthetic approach to kingianin A based on biosynthetic speculation†
Abstract
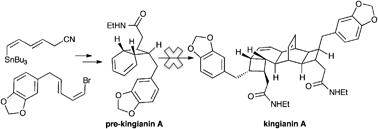
A synthetic approach towards the structurally complex dimer, kingianin A is reported. The strategy involved a cascade of complexity generating reactions, inspired through biosynthetic speculation. A concise

 Please wait while we load your content...
Please wait while we load your content...