New insight on 2-naphthylmethyl (NAP) ether as a protecting group in carbohydrate synthesis: a divergent approach towards a high-mannose type oligosaccharide library†‡
Abstract
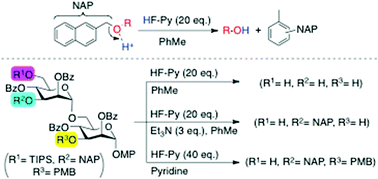
A new method for selective cleavage of 2-naphthylmethyl (NAP) ether utilizing 10–20 molar excess of HF/
- This article is part of the themed collection: Glycochemistry & glycobiology

 Please wait while we load your content...
Please wait while we load your content...