Control of selectivity in the generation and reactions of oxonium ylides†
Abstract
Dirhodium catalyzed reactions of
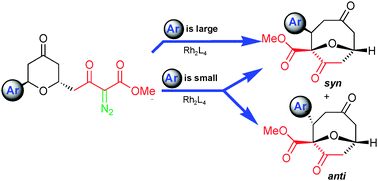
- This article is part of the themed collection: New advances in catalytic C–C bond formation via late transition metals

 Please wait while we load your content...
Please wait while we load your content...