A highly active and selective palladium pincer catalyst for the formation of α-aryl ketonesvia cross-coupling†‡
Abstract
Several air and moisture stable Pd(II) pincer complexes were synthesized via oxidative addition of Pd(0) to novel PheBox pincer
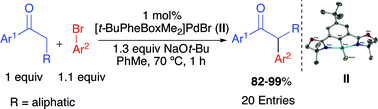
- This article is part of the themed collection: New advances in catalytic C–C bond formation via late transition metals

 Please wait while we load your content...
Please wait while we load your content...