Autocatalytic formation of fluorinated ferrocenophanes from 1,1′-bis(trifluorovinyl)ferrocene†
Abstract
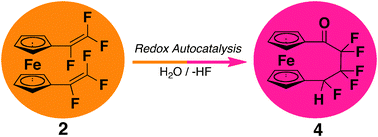
1,1′-Bis(trifluorovinyl)ferrocene is obtained in excellent yields from a Negishi type coupling reaction. Under certain redox conditions and in the presence of a suitable nucleophile, the highly reactive compound undergoes a transformation to fluorinated ferrocenophanes. Intra and intermolecular [2+2]-cycloaddition products are formed upon heating.

 Please wait while we load your content...
Please wait while we load your content...