A cascade Heck–Aldol–Heck reaction by a combination of transition-metal catalysis and aminocatalysis†
Abstract
An unprecedented cascade Heck–Aldol–![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in one process by a combination of palladium(0) catalysis and aminocatalysis. Various aryl iodides could perform the cascade reaction with readily available
C double bond in one process by a combination of palladium(0) catalysis and aminocatalysis. Various aryl iodides could perform the cascade reaction with readily available
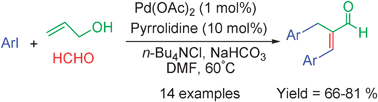

 Please wait while we load your content...
Please wait while we load your content...