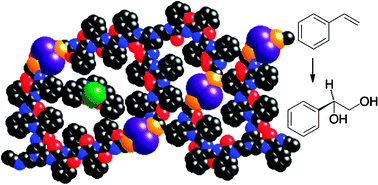
An achiral manganese salen catalyst encapsulated in a peptidic phosphonate homochiral solid for the enantioselective formation of diols by consecutive epoxidation and hydration reactions†
Abstract
An insoluble, porous, amorphous, homochiral material based on a

 Please wait while we load your content...
Please wait while we load your content...