An investigation into the origin of the dramatically reduced reactivity of peptide-prolyl-thioesters in native chemical ligation†
Abstract
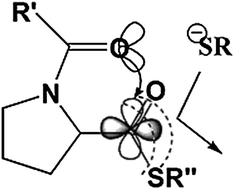
The low reactivity of peptide-prolyl-thioesters in native chemical ligation is not due to steric effects at the β-carbon, but rather to the presence of a

 Please wait while we load your content...
Please wait while we load your content...