Acetoxypalladation of unactivated alkynes and capture with alkenes to give 1-acetoxy-1,3-dienes taking dioxygen as terminal oxidant†
Abstract
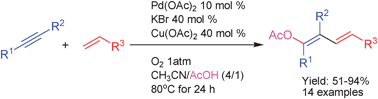
A new and general protocol for the synthesis of 1-acetoxy-1,3-dienes by an acetoxypalladation/Heck cross-coupling/β-H elimination tandem process is described in which dioxygen is the terminal oxidant. Electron-rich and electron-deficient
- This article is part of the themed collection: New advances in catalytic C–C bond formation via late transition metals

 Please wait while we load your content...
Please wait while we load your content...