Preparation of benzolactams by Pd(ii)-catalyzed carbonylation of N-unprotected arylethylamines†
Abstract
An unprecedented NH2-directed Pd(II)-catalytic
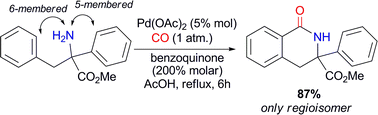
- This article is part of the themed collection: New advances in catalytic C–C bond formation via late transition metals

 Please wait while we load your content...
Please wait while we load your content...