Mimicking multipass transmembrane proteins: synthesis, assembly and folding of alternating amphiphilic multiblock molecules in liposomal membranes†‡
Abstract
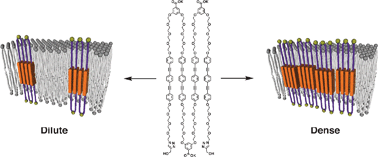
Alternating amphiphilic multiblock molecules 1–4, involving fluorescent hydrophobic units, were designed as mimics for multipass transmembrane

 Please wait while we load your content...
Please wait while we load your content...