Nickel-catalysed cross-coupling reaction of aryl(trialkyl)silanes with aryl chlorides and tosylates†
Abstract
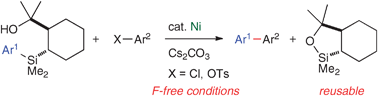
Using highly stable, readily accessible, and recyclable 2-(2-hydroxyprop-2-yl)cyclohexyl-substituted arylsilanes activated by a mild
- This article is part of the themed collection: New advances in catalytic C–C bond formation via late transition metals

 Please wait while we load your content...
Please wait while we load your content...