The “GI dissolution” method: a low volume, in vitro apparatus for assessing the dissolution/precipitation behaviour of an active pharmaceutical ingredient under biorelevant conditions
Abstract
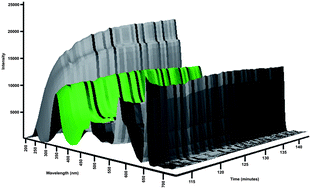
This paper describes a low volume, in vitro apparatus for investigating the dissolution and

 Please wait while we load your content...
Please wait while we load your content...