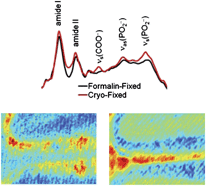
Understanding biochemical mechanisms and changes associated with disease conditions and, therefore, development of improved clinical treatments, is relying increasingly on various biochemical mapping and imaging techniques on tissue sections. However, it is essential to be able to ascertain whether the sampling used provides the full biochemical information relevant to the disease and is free from artefacts. A multi-modal micro-spectroscopic approach, including FTIR imaging and PIXE elemental mapping, has been used to study the molecular and elemental profile within cryofixed and formalin-fixed murine brain tissue sections. The results provide strong evidence that amino acids, carbohydrates, lipids, phosphates, proteins and ions, such as Cl− and K+, leach from tissue sections into the aqueous fixative medium during formalin fixation of the sections. Large changes in the concentrations and distributions of most of these components are also observed by washing in PBS even for short periods. The most likely source of the chemical species lost during fixation is the extra-cellular and intra-cellular fluid of tissues. The results highlight that, at best, analysis of formalin-fixed tissues gives only part of the complete biochemical “picture” of a tissue sample. Further, this investigation has highlighted that significant lipid peroxidation/oxidation may occur during formalin fixation and that the use of standard histological fixation reagents can result in significant and differential metal contamination of different regions of tissue sections. While a consistent and reproducible fixation method may be suitable for diagnostic purposes, the findings of this study strongly question the use of formalin fixation prior to spectroscopic studies of the molecular and elemental composition of biological samples, if the primary purpose is mechanistic studies of disease pathogenesis.

 Please wait while we load your content...
Please wait while we load your content...