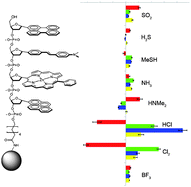
We describe a new molecular design for sensing toxic gases in air that employs DNA-polyfluorophores as reporters. In these polyfluorophores (ODFs), the DNA backbone is used as a scaffold to arrange several fluorescent aromatic hydrocarbons/heterocycles as DNA base replacements in a photophysically interacting stack. A library of 256 different tetramer ODFs was constructed on PEG-polystyrene beads and tested for optical responses to a set of toxic gases, SO2, H2S, MeSH, NH3, NHMe2, HCl, Cl2, and BF3. A set of 15 responding sequences was resynthesized, characterized, and cross-screened under a microscope against all eight gases at 1000 ppm. Responses were measured by changes in fluorescence wavelength and intensity, which were quantified as Δ(R, G, B, L) data from bead images. The data show a large range of responses, including lighting up, quenching, and color changes; remarkably, some single sensors showed all three types of responses to the varied analytes. Statistical methods were used to identify small sets of only three chemosensors that could be used to distinguish all eight analytes. The results establish that ODFs on surfaces can bind and report on small, simple gas molecules with highly varied responses. The DNA-like structure of the sensor molecules confers a number of potential advantages including simple synthesis, high diversity, and rapid sensor discovery.

 Please wait while we load your content...
Please wait while we load your content...