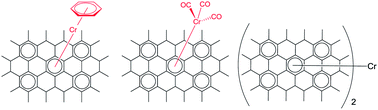
We explore the organometallic chemistry of graphitic materials and demonstrate the η6-complexation reactions of chromium with graphene, graphite and carbon nanotubes. All of these extended periodic π-electron systems exhibit some degree of reactivity toward the reagents Cr(CO)6 and (η6-benzene)Cr(CO)3, and we are able to demonstrate the formation of (η6-arene)Cr(CO)3 or (η6-arene)2Cr, where arene = single-walled carbon nanotubes (SWNT), exfoliated graphene (XG), epitaxial graphene (EG) and highly-oriented pyrolytic graphite (HOPG). We report the ATR-IR, Raman spectroscopy, XPS and chemistry of these new organometallic species and we observe clearly understandable trends in the chemistry and stability of the complexes based on curvature and surface presentation. For example, the SWNTs are the least reactive presumably as a result of the effect of curvature on the formation of the hexahapto bond; in the case of HOPG, (η6-HOPG)Cr(CO)3 was isolated while the exfoliated graphene samples were found to give both (η6-graphene)2Cr, and (η6-graphene)Cr(CO)3 structures. We report simple and efficient routes for the mild decomplexation of the graphene–chromium complexes which appears to restore the original pristine graphene state; exposure of the samples to white light in a solution of acetonitrile or the use of selected ligand competition reactions bring about a clean reversal of the metal complexation reactions and provide an independent proof of structure for the reaction products. This study represents the first example of the use of graphene as a ligand and is expected to expand the scope of graphene chemistry in connection with the application of this material in organometallic catalysis, where graphene can act as an electronically conjugated catalyst support.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...