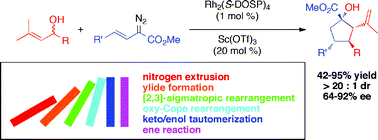
A domino sequence has been developed between vinyldiazoacetates and racemic allyl alcohols, involving five distinct steps. The sequence generates highly functionalized cyclopentanes with four new stereogenic centers as single diastereomers in 64–92% ee. The first step is a rhodium-catalyzed oxygen ylide formation, which is then followed by a [2,3]-sigmatropic rearrangement, an oxy-Cope rearrangement, a keto/enol tautomerization, and then finally a carbonyl ene reaction. With appropriate substrates, a further silyl deprotection and a 6-exo-trigcyclization can be added to the domino process.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...