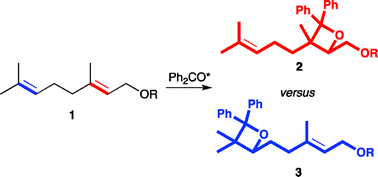
The Paternò–Büchi (PB) reaction of geraniol derivatives 1, which contain allylic alcohol functionality and unfunctionalized double bonds, with benzophenone was investigated to see the effect of the hydroxyl group on the regioselectivity of the oxetane formation, i.e., 2/3. At low concentration of geraniol (1a), oxetanes 2a and 3a were formed in a ratio of 2a/3a = ca. 50/50. The oxetane 2a is derived from the PB reaction at the allylic alcohol moiety, whereas the PB reaction at the unfunctionalized double bond produces the oxetane 3a. The PB reaction of the hydroxy-protected methyl ether 1b and acetate 1c gave selectively oxetanes 3b,c derived from the reaction at the more nucleophilic double bond, 2/3 ∼ 15/85. The hydroxyl-group effect was found to be small, but apparently increased the formation of 2a in the PB reaction with geraniol (1a).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...