The reaction of S-nitrosocaptopril (NOcap) formation was studied in both aqueous acid and basic medium. Captopril (cap) reacts rapidly with nitrous acid in strong acid medium to give the stable—in the timescale of the experiments—NOcap. The kinetic study of the reaction involving the use of stopped-flow, shows that at low sodium nitrite (nit) concentration, the reaction is first-order in both [nit], [H+], and is strongly catalysed by Cl− or Br− (= X−): rate = (k3 + k4[X−])[H+][nit][cap]. In aqueous buffered solution of acetic acid–acetate the reaction rate is much slower and the decomposition of NOcap was observed; however, the rate of NOcap decay is more than 30-fold slower than its formation. In aqueous basic medium of carbonate–hydrogen carbonate buffer, as well as in alkaline medium, the kinetics of the nitroso group (NO) transfer from tert-butyl nitrite (tBN) to cap was studied using either conventional or stopped flow methods. In mild basic medium, the NOcap decomposes. The NOcap formation is first-order in both tBN and cap concentrations, and the reaction rate increases with pH until to, approximately, pH 11.5, above which value it becomes pH independent or even invariable with the [OH−]. Kinetic results show that the thiolate ion of cap is the reactive species. In fact, the presence of anionic micelles of sodium dodecyl sulfate (SDS) inhibits the reaction due to the separation of the reagents; whereas, cationic micelles of tetradecyltrimethylammonium bromide (TTABr) catalyse the reaction at low surfactant concentration due to reagents concentration in the small volume of the micelle. The rate equation is: rate = kfKSH[cap][tBN]/(KSH + [H+]). The rate of NOcap decomposition in mild basic medium is first-order in both [cap] and [NOcap], and decreases on increasing pH; but, in alkaline medium the NOcap is stable within the timescale of the experiments. Based on the results, the NOcap decomposition yields the disulfide compound that is formed in the nucleophilic attack of the –SH group of cap to the sulfur electrophilic center of NOcap, –S–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The resulting rate equation is: rate = kd[H+][cap][NOcap]/(KSH + [H+]).
O. The resulting rate equation is: rate = kd[H+][cap][NOcap]/(KSH + [H+]).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The resulting rate equation is: rate = kd[H+][cap][NOcap]/(KSH + [H+]).
O. The resulting rate equation is: rate = kd[H+][cap][NOcap]/(KSH + [H+]).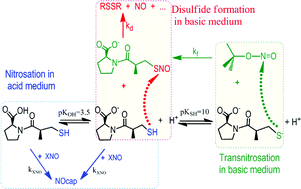

 Please wait while we load your content...
Please wait while we load your content...