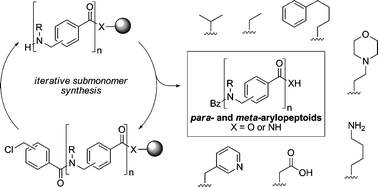
Efficient and versatile COMU-mediated solid-phase submonomer synthesis of arylopeptoids (oligomeric N-substituted aminomethyl benzamides)†
Abstract
The development of a highly efficient methodology for solid-phase synthesis of para- and meta-arylopeptoids (oligomeric N-substituted aminomethyl benzamides) with free acids or free
 Please wait while we load your content...
Please wait while we load your content...