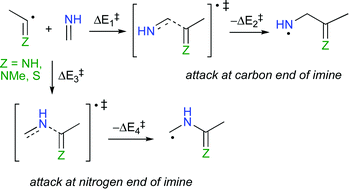
Ab initio and DFT calculations reveal that both imidoyl and thioyl radicals add to the nitrogen end of methanimine through simultaneous SOMO-π*imine, SOMO-πimine, SOMO-LPN and π*radical-LPN interactions between the radical and the imine. At the CCSD(T)/cc-pVDZ//BHandHLYP/cc-pVTZ level of theory, barriers of 13.8 and 26.1 kJ mol−1 are calculated for the attack of the methylimidoyl radical at the carbon- and nitrogen- end of methanimine, respectively, indicating that the imidoyl radial has a preference for addition to the nitrogen end of imine. On the other hand, barriers of 25.1 and 13.4 kJ mol−1 are calculated at the same level of theory for the addition reaction of the methanethioyl radical at the carbon- and nitrogen- end of methanimine, respectively. Natural bond orbital (NBO) analysis at the BHandHLYP/6-311G** level of theory reveals that SOMO-π*imine, SOMO-πimine, SOMO-LPN and π*radical-LPN interactions are worth 111, 89, 115 and 17 kJ mol−1, respectively, in the transition state (4) for the reaction of methylimidoyl radical at the nitrogen end of methanimine; similar interactions are observed for the chemistry involving all the radicals studied here. These multi-component interactions are responsible for the unusual motion vectors associated with the transition states involved in these reactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...