Synthesis and biological evaluation of 2,3-bis(het)aryl-4-azaindole derivatives as protein kinase inhibitors†
Abstract
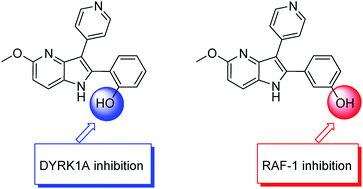
The synthesis of several novel 4-azaindoles was carried out by novel Fischer reaction which offers as a main advantage, the synthesis of the bisfunctionalized 4-azaindolic building block in one step. The final compounds were evaluated on a panel of 5 kinases in order to evaluate their selectivity and on 7 cancer cell lines to determine their cytotoxic effects. RAF-1 and DYRK1A inhibitions were found, docking studies explain fully the results.

 Please wait while we load your content...
Please wait while we load your content...