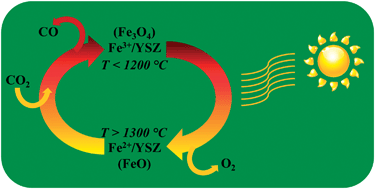
Ferrites are promising materials for enabling solar-thermochemical cycles. Such cycles utilize solar-thermal energy for the production of hydrogen from water, or carbon monoxide from carbon dioxide. Mixing ferrites with zirconia or yttria-stabilized zirconia (YSZ) greatly improves the cyclability of the ferrites and enables a move away from powder to monolithic systems. This synergistic effect is only partially understood. In order to unravel the underlying mechanisms of the effect and to understand the evolution of thermochemically active phases, we have studied the behaviour of iron oxides co-sintered with 8YSZ (8 mol% Y2O3) using in operando X-ray diffraction and thermogravimetric analysis at temperatures up to 1500 °C and under environments representative of those present in a thermochemical cycle. The solubility of iron oxide in 8YSZ measured by XRD at room temperature, following calcination to 1500 °C in air, was 9.4 mol% Fe. The solubility increased to at least 10.4 mol% Fe when heated between 800 and 1000 °C under inert (He) atmosphere. Furthermore iron was found to migrate in and out of the 8YSZ phase as the temperature and oxidation state of the iron changed. In samples containing insoluble iron (i.e., containing >9.4 mol% Fe) stepwise heating to 1400 °C under helium caused reduction of Fe2O3 (hematite) to Fe3O4 (magnetite) to FeO (wüstite). This gradual thermal reduction from hematite to wüstite was accompanied by evolution of oxygen. The wüstite remained stable upon cooling to room temperature in the helium environment, although after multiple consecutive cycles some of the wüstite was observed to disproportionate to Fe metal and magnetite. Exposure of the wüstite-containing material to CO2 at 1100 °C enabled re-oxidation of the wüstite to magnetite with evolution of CO. Thermogravimetric analysis during thermochemical cycling of materials with iron oxide contents between 1.8 and 27.6 mol% Fe showed that samples with mostly dissolved iron utilized a greater proportion of the iron atoms present than did samples possessing a significant fraction of un-dissolved iron oxides.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...