Benefits of N for O substitution in polyoxoanionic electrode materials: a first principles investigation of the electrochemical properties of Li2FeSiO4−yNy (y = 0, 0.5, 1)†
Abstract
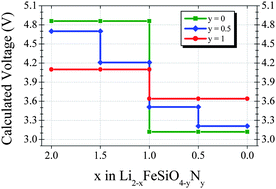
First principles calculations have been used to investigate the effect of N for O substitution on the electrochemical properties of Li2FeSiO4. Within the Li2FeSiO4 structure, hypothetical models of the N-substituted Li2FeSiO3N and Li2FeSiO3.5N0.5 have been analyzed. The computational results indicate that the lithium deinsertion voltage associated to the Fe3+/Fe4+ redox couple can be decreased by N substitution (4.86 V in Li2FeSiO4, 4.7 V in Li2FeSiO3.5N0.5 and 4.1 V in Li2FeSiO3N). The high theoretical specific capacity of Li2FeSiO4 (330 mA h g−1) could be retained in N-substituted silicates thanks to the oxidation of N3− anions. The redox activity of N ions is observed in a voltage range of ca. 3.5–4.2 V. In the light of the potential benefits of N substitution for O experimental work is encouraged, in particular to investigate the reversibility and overpotential of the N redox reaction.
- This article is part of the themed collection: Advanced Materials for Lithium Batteries

 Please wait while we load your content...
Please wait while we load your content...