Direct solid-state synthesis and large-capacity anode operation of Li3−xFexN
Abstract
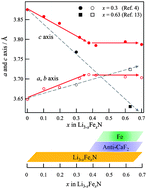
A direct solid-state synthesis route to Li3−xFexN (x < 0.4) was developed and its electrochemical properties as an anode material for lithium-ion batteries were investigated. In order to stabilize the lower valence state of Fe(I) in the layered structure of Li1−xFexN against Fe(III) in the competent phase of anti-fluorite Li3FeN2, an argon flow was used during the sintering process instead of the
- This article is part of the themed collection: Advanced Materials for Lithium Batteries

 Please wait while we load your content...
Please wait while we load your content...