Electrochemical characterization of lithium 4,4′-tolane-dicarboxylate for use as a negative electrode in Li-ion batteries†‡
Abstract
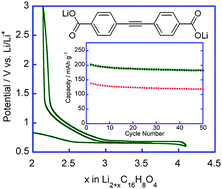
- This article is part of the themed collection: Celebrating the 70th birthday of Professor Fred Wudl

 Please wait while we load your content...
Please wait while we load your content...